As discussed on the gas properties page, there are two ways to look at a fluid; from the large, macro scale properties of the fluid that we can measure, and from the small, micro scale of the molecular motion and interaction. On this page, we will consider Bernoulli's equation from both standpoints.
Macro Scale Derivation
Thermodynamics is the branch of science which describes the macro scale properties of a fluid. One of the principle results of the study of thermodynamics is the conservation of energy; within a system, energy is neither created nor destroyed but may be converted from one form to another. We shall derive Bernoulli's equation by starting with the conservation of energy equation. The most general form for the conservation of energy is given on the Navier-Stokes equation page. This formula includes the effects of unsteady flows and viscous interactions. Assuming a steady, inviscid flow we have a simplifiedconservation of energy equation in terms of the enthalpy of the fluid:
Assuming no heat transfer into the fluid, and no work done by the fluid, we have:
It is important when applying any equation that you are aware of the restrictions on its use; the restrictions usually arise in the derivation of the equation when certain simplifying assumptions about the nature of the problem are made. If you ignore the restrictions, you may often get an incorrect "answer" from the equation. For instance, this form of the equation was derived while assuming that the flow was incompressible, which means that the speed of the flow is much less than the speed of sound. If you use this form for a supersonic flow, the answer will be wrong.
Molecular Scale Derivation
We can make another interpretation of the equation by considering the motion of the gas molecules. The molecules within a fluid are in constant random motion and collide with each other and with the walls of an object in the fluid. The motion of the molecules gives the molecules a linear momentum and the fluid pressure is a measure of this momentum. If a gas is at rest, all of the motion of the molecules is random and the pressure that we detect is the total pressure of the gas. If the gas is set in motion or flows, some of the random components of velocity are changed in favor of the directed motion. We call the directed motion "ordered," as opposed to the disordered random motion.
We can associate a "pressure" with the momentum of the ordered motion of the gas. We call this pressure the dynamic pressure. The remaining random motion of the molecules still produces a pressure called the static pressure. At the molecular level, there is no distinction between random and ordered motion. Each molecule has a velocity in some direction until it collides with another molecule and the velocity is changed. But when you sum up all the velocities of all the molecules you will detect the ordered motion. From a conservation of energy and momentum, the static pressure plus the dynamic pressure is equal to the original total pressure in a flow (assuming we do not add or subtract energy in the flow). The form of the dynamic pressure is the density times the square of the velocity divided by two.
Applications of Bernoulli's Equation
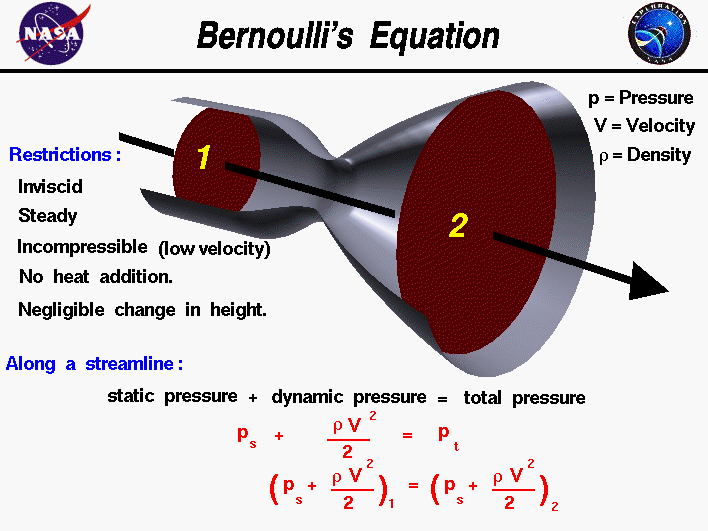
The fluids problem shown on this slide is low speed flow through a tube with changing cross-sectional area. For a streamline along the center of the tube, the velocity decreases from station one to two. Bernoulli's equation describes the relation between velocity, density, and pressure for this flow problem. Since density is a constant for a low speed problem, the equation at the bottom of the slide relates the pressure and velocity at station two to the conditions at station one.









0 comments:
Post a Comment